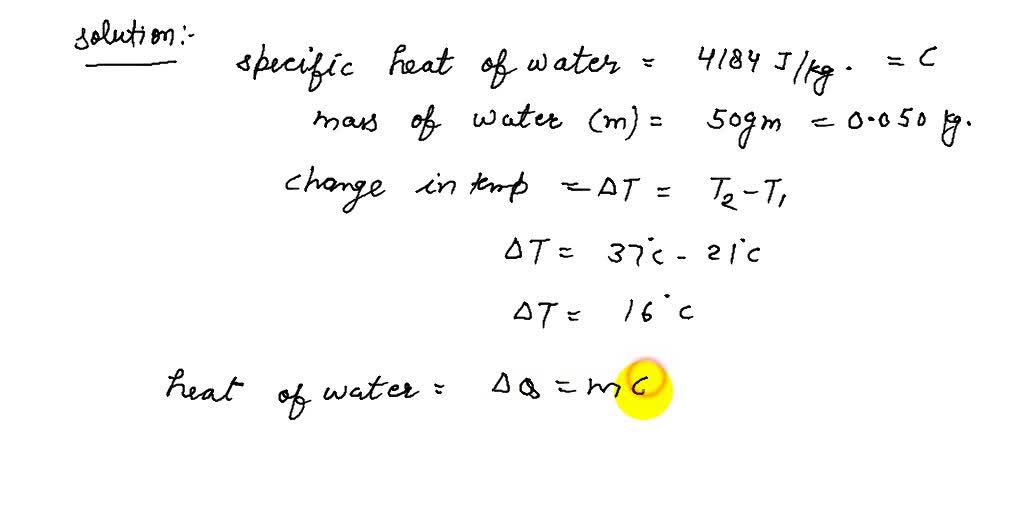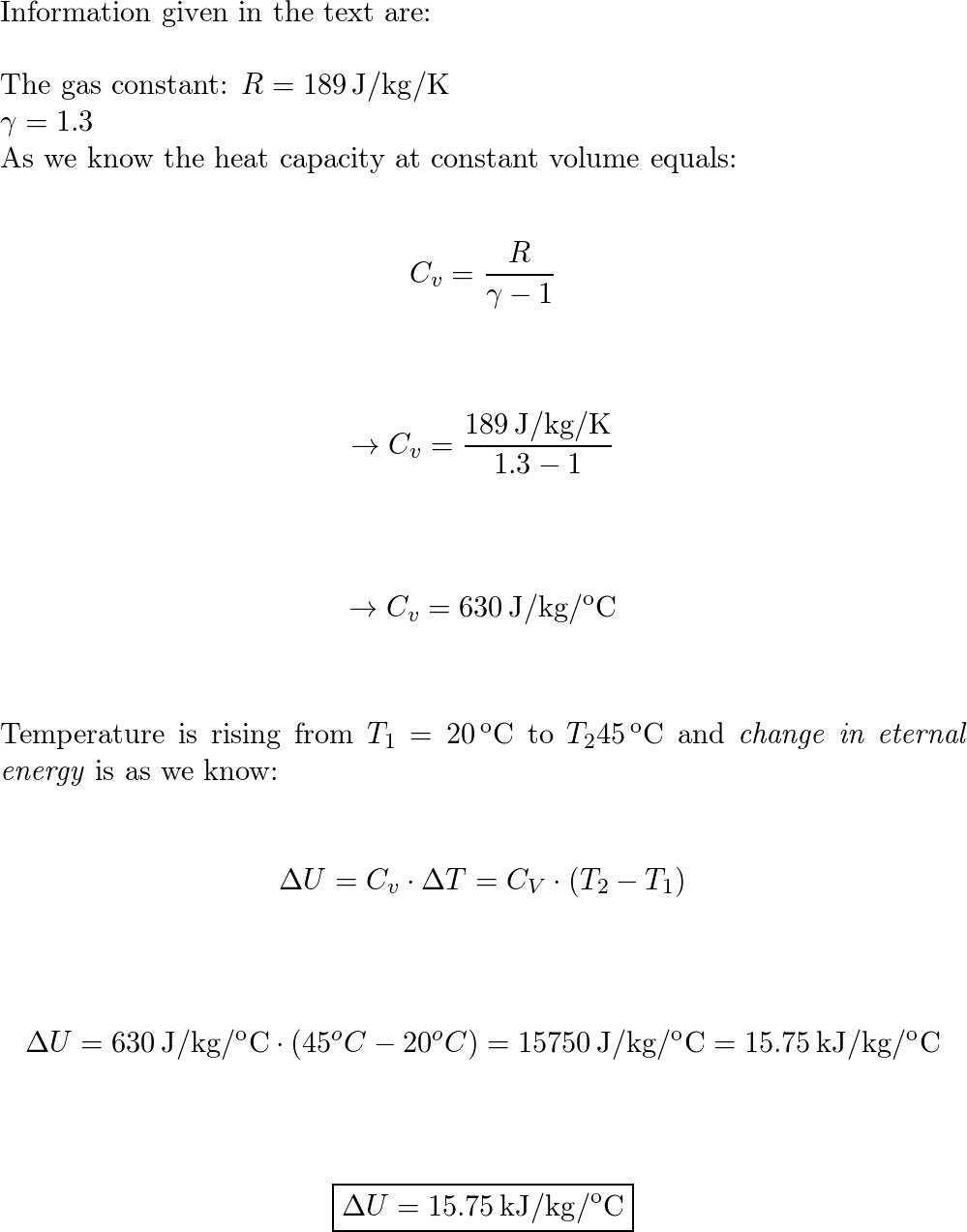For a gas the differce between the two specific heat is `4150 J//kg K`. What is the specific heat at - YouTube
Cheat calculations Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

A well-insulated piston-cylinder device is shown in the figure. The piston-cylinder contains m_{g}=1 kg of gas with c_{v}=620 J/kg-k (assume constant and ideal-gas) and R=410 J/kg-K. The initial pressure of the gas

A calorimeter of mass `0.2 kg` and specific heat `900 J//kg-K`. Containing `0.5 kg` of a liquid of - YouTube

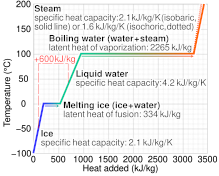
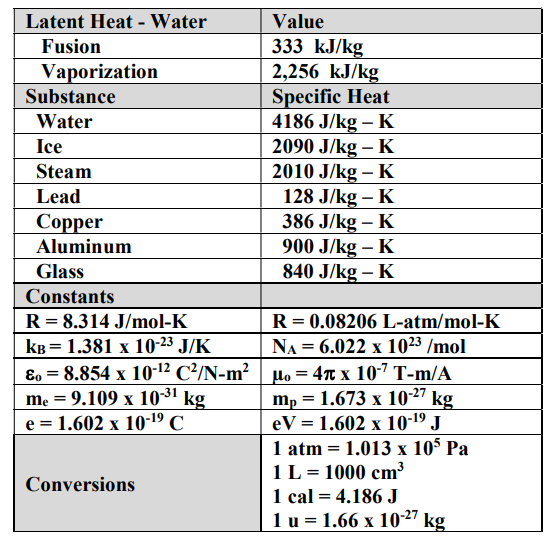
SOLVED: Useful Constants Cice–2050 J/(kg:*C) Cwater` =4184 J/(kg:"€ Csteam=1996 J /(kg: Ccopper =390 J/(kg:" Lfus, waler =334 kJ/kg Lvap; water =2257 kJ/kg 1 atm =Ix105 Pa R=8.314 J/(mol K)
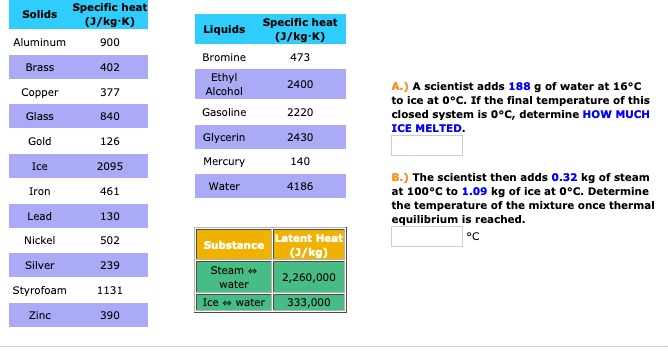
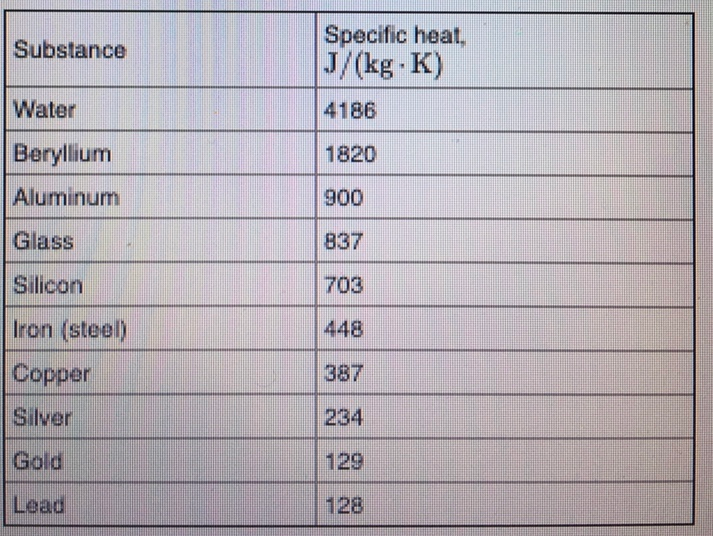
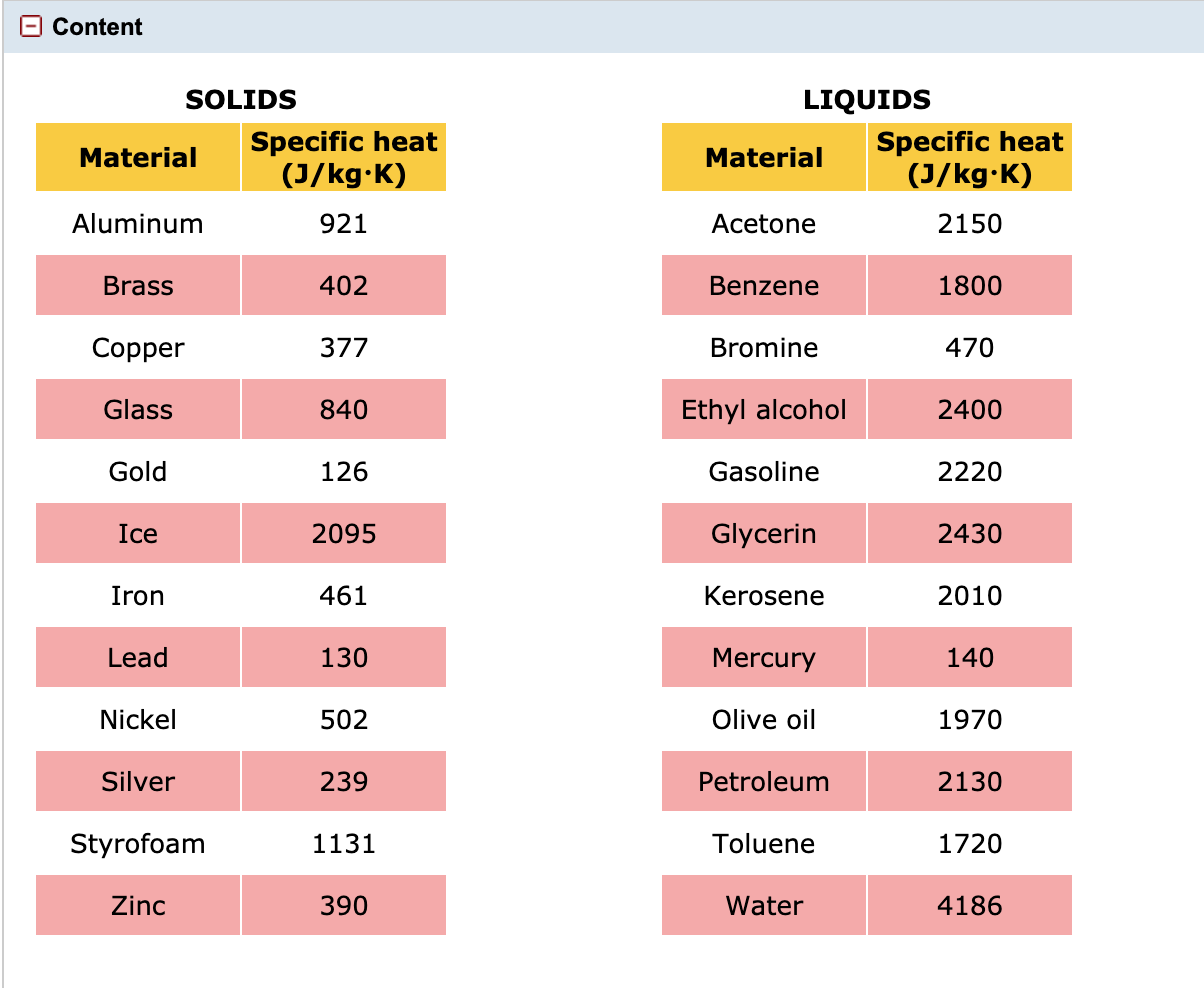
SOLVED: Specific heat Solids (J/kg K) Specific heat Liquids (J/kg K) Bromine 473 Ethyl 2400 Alcohol Aluminum 900 Brass 402 scientist adds 188 water at 16'C at 0PC: If the final temperature

Air ( ? = 1.4, R= 287 J/kg.K) at 100 kPa and 290K is flowing in a constant area tube with a velocity of 100 m/s. Suddenly the end of the tube

Polynomial constants for specific heat at constant pressure c p (J/kg.K). | Download Scientific Diagram

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity
![Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram](https://www.researchgate.net/profile/Radoslaw-Wach/publication/256115072/figure/tbl1/AS:755605098729495@1557161702871/Specific-heat-capacity-C-p-J-kgK-of-small-samples-E-F-and-G-at-various_Q640.jpg)
Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram

Polynomial constants for specific heat at constant pressure c p (J/kg.K). | Download Scientific Diagram