Marketing authorization and licensing of medicinal products in EU: Regulatory aspects - ScienceDirect
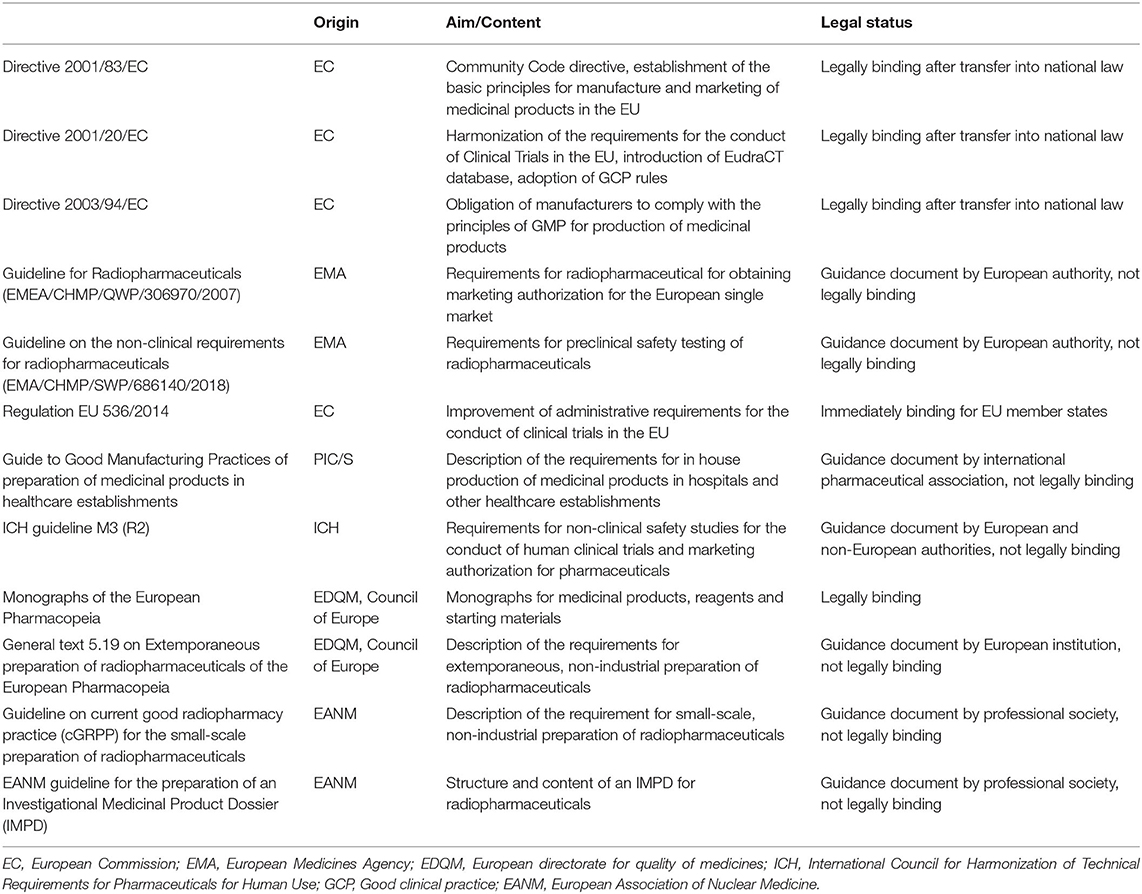
Frontiers | Emerging Radionuclides in a Regulatory Framework for Medicinal Products – How Do They Fit?

The EU regulatory network and emerging trends – a review of quality, safety and clinical development programmes - GaBI Journal

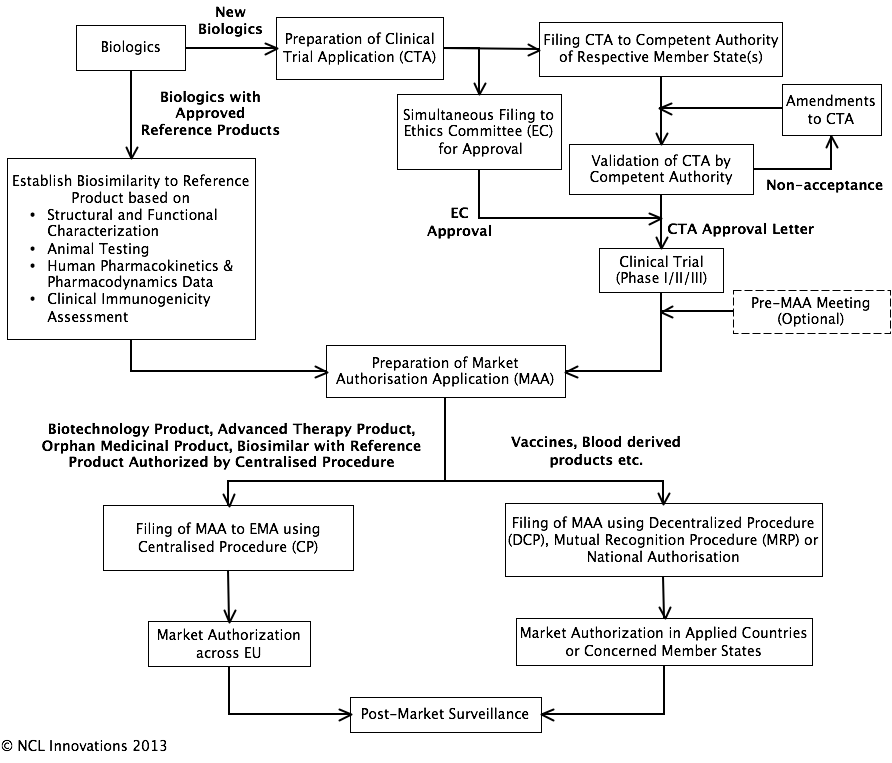
Marketing authorization and licensing of medicinal products in EU: Regulatory aspects - ScienceDirect

Good Manufacturing Practice (GMP) Guidelines: The Rules Governing Medicinal Products in the European Union, EudraLex Volume 4 Concise Reference by Allport-Settle, Mindy J - Amazon.ae
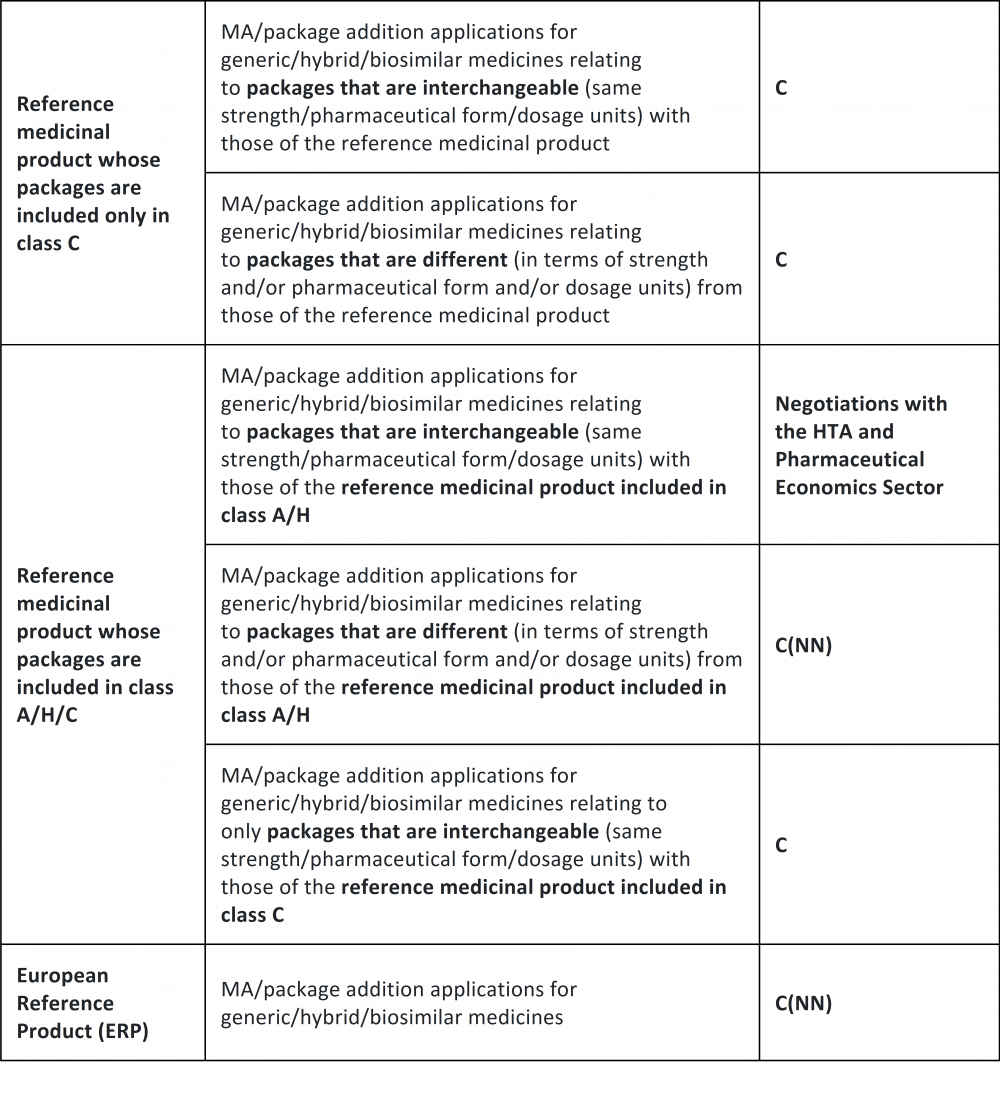
The Italian Medicines Agency provides additional information on the new simplified classification procedure for generics and biosimilars - Portolano Cavallo

Inês Alves - Member of the Committee for Orphan Medicinal Products - COMP - European Medicines Agency | LinkedIn
ad hoc working group on validation issues/national requirements common grounds for invalidation/delaying validation
The european substance reference system (EU - SRS) release strategy widely supported by the community - UNICOM

Biotherapeutic products in the European Pharmacopoeia: have all challenges been tackled? - GaBI Journal

Good Manufacturing Practice (GMP) Guidelines: The Rules Governing Medicinal Products in the European Union, Eudralex Volume 4 Concise Reference (Paperback or Softback) by Allport-Settle, Mindy J.: New Paperback or Softback (2009) | BargainBookStores